Learn about EGFR-positive NSCLC
If you have non-small cell lung cancer (NSCLC) and your doctor told you that you have an EGFR mutation, we will help you understand what this means and how it impacts your treatment options. This will help you talk with your doctor to decide the best treatment options for you.
What is EGFR?
EGFR stands for epidermal growth factor receptor. EGFR is a naturally occurring protein on cells that helps them grow. Certain changes (mutations) in the EGFR protein can cause the cells to grow too much, which can cause cancer.
The EGFR mutation was the first “actionable” (targetable) biomarker discovered in people with lung cancer, meaning it was the first to have a targeted treatment (therapy) that finds and attacks the driver mutation to stop cancer cell growth.
What is a biomarker?
A biomarker is a substance that doctors can measure in tissues, blood, or other body fluids like spit. In NSCLC, biomarkers are changes in a cell’s genes (building blocks of a cell’s DNA) or proteins that cause the cancer to grow. These mutations are called “driver mutations” because they cause (or “drive”) cancer to grow.
Who gets EGFR-positive NSCLC?
Approximately 15% of patients with NSCLC in the US and 35% of patients from East Asia have tumors with an EGFR driver mutation.
Regardless of the patient's ethnicity, people with EGFR driver mutations are most often females, nonsmokers, and/or young adults (EGFR mutations are present in roughly 50% of lung cancers in young adults). Most commonly, these patients have lung adenocarcinoma.
How do I know if I have an EGFR mutation?
After being diagnosed with lung cancer, ask your doctor for comprehensive biomarker testing. Comprehensive biomarker testing checks your lung cancer for a full list of biomarkers, including EGFR, that doctors have linked to NSCLC. It is important to know if you have any biomarkers to help decide what your treatment options might be – different treatments work better on cancers with certain biomarkers.
There are two types of biomarker testing: tissue biopsy and liquid biopsy. During a tissue biopsy, doctors will use a sharp tool to get a sample of tissue from your lung cancer. During a liquid biopsy, doctors will take a sample of blood to look for cancer cells from your lung cancer tumor circulating in your blood. Currently, tissue biopsies are the standard of care if you have enough tissue, but liquid biopsies are showing potential to be as accurate as tissue biopsies in the future.
Are there different types of EGFR mutations?
Yes, there are a number of different types of EGFR mutations. Each of the different types of EGFR mutations affects the DNA differently. For example, some of the EGFR mutations cause something to be missing from the DNA, and they are called “deletions.” Some of the EGFR mutations cause something to be added to the DNA, and they are called “insertions.” Other EGFRs mutations cause something to be changed, and they are called “point mutations.”
The most common EGFR mutations are exon 19 deletions and L858R point mutations.
How do doctors treat EGFR-positive NSCLC?
You will work with your doctor to decide on the treatment that is best for you, based on your stage and type of EGFR lung cancer.
Currently, EGFR-positive NSCLC is most often treated with a targeted therapy. A targeted therapy is a type of treatment that finds and attacks certain parts of cancer cells and the signals sent to cancer cells that cause them to grow uncontrolled. They prevent the growth of cancer cells only, and do not harm healthy cells.
Most EGFR-positive lung cancers respond best to targeted therapies with tyrosine kinase inhibitors (TKIs). Tyrosine kinases are proteins that allow signals to be sent from one cell to the other. These signals are sent to tyrosine kinase receptors on cells.
When you have an EGFR mutation, the tyrosine kinases send messages to the receptors that tell the cancer cells to grow and spread. However, when you receive a TKI, the drug blocks the signal from reaching the receptor. This means the cancer cells do not continue to grow and spread and the cancer is kept in check.
There are currently eight FDA-approved EGFR TKIs. All are approved for EGFR exon 19 deletion and exon 21 (L858R) substitution mutations, with the exception of mobocertinib (ExkivityTM) and sunvozertinib (Zegfrovy®), which are approved for EGFR exon 20 insertion mutations. Afatinib (Gilotrif®) and osimertinib (Tagrisso®) have additional indications as well.
- Afatinib (Gilotrif®): Approved for first-line treatment of patients with metastatic NSCLC whose tumors have EGFR non-resistant mutations, as detected by an FDA-approved test. (The most common of these are the exon 19 deletion and the exon 21 (L858R) substitution mutations. The rarer mutations are S768L, L861Q, and G719X.)
- Dacomitinib (Vizimpro®): Approved for first-line treatment of patients with metastatic NSCLC whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations, as detected by an FDA-approved test
- Erlotinib (Tarceva®): Approved for the treatment of patients with EGFR-positive metastatic NSCLC. This includes patients whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations, as detected by an FDA-approved test, who are receiving first-line or maintenance treatment, or second- or subsequent-line treatment after progression following at least one prior chemotherapy regimen. Erlotinib (Tarceva®) is also approved in combination with ramucirumab (Cyramza®), an angiogenesis inhibitor, for the first-line treatment of metastatic NSCLC with EGFR exon 19 deletions or exon 21 (L858R) substitution mutations
- Gefitinib (Iressa®): Approved for the first-line treatment of patients with metastatic NSCLC whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations, as detected by an FDA-approved test
- Lazertinib (LazcluzeTM): Approved in combination with amivantamab for the first-line treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test.
- Mobocertinib (ExkivityTM): Approved for the treatment of adult patients with locally advanced or metastatic EGFR exon 20 insertion-mutant NSCLC, as detected by an FDA-approved test, and who have received prior platinum-based chemotherapy
Following discussions with the US Food and Drug Administration, EXKIVITY will be officially withdrawn from the market in the United States no later than March 2024. EXKIVITY remains available to prescribe until the official withdrawal; moreover, after withdrawal patients will be able to maintain access through an established compassionate use program. All current patients on the drug whom Takeda has contact information for will have received an official communication. For more information, please visit www.exkivity-update.com. - Osimertinib (Tagrisso®):
- Approved for first-line treatment of patients with metastatic NSCLC whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations, as detected by an FDA-approved test.
- Approved for second-line treatment of patients with metastatic NSCLC whose tumors are EGFR T790M-positive, as detected by an FDA-approved test, and whose disease has progressed on or after EGFR TKI therapy.
- Approved for the treatment of adult patients with locally advanced, unresectable (stage III) NSCLC whose disease has not progressed during or following concurrent or sequential platinum-based chemoradiation therapy and whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations, as detected by an FDA-approved test.
Note: The US FDA granted approval for the use of osimertinib (Tagrisso®) as adjuvant therapy after surgical removal of a tumor in adult patients with stages 1b to IIIa NSCLC whose tumors are mostly nonsquamous and have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations, as detected by an FDA-approved test. - Osimertinib (Tagrisso®) is also approved in combination with pemetrexed and platinum-based chemotherapy for the first-line treatment of adult patients with locally advanced or metastatic NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations, as detected by an FDA-approved test.
- Sunvozertinib (Zegfrovy®): Approved for adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy.
There are currently two FDA-approved treatments that are not TKIs for the treatment of EGFR driver mutations. Amivantamab-vmjw (RybrevantTM) is a bispecific antibody. Datopotamab deruxtecan-dlnk (Datroway®) is an antibody-drug conjugate.
- Amivantamb-vmjw (RybrevantTM):
- Approved for the treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy.
- Approved in combination with carboplatin and pemetrexed for the first-line treatment of adult patients with locally advanced or metastatic NSCLC with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an FDA-approved test.
- Approved in combination with lazertinib for the first-line treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test.
- Approved in combination with carboplatin and pemetrexed for the treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations, whose disease has progressed on or after treatment with an EGFR tyrosine kinase inhibitor.
- Datopotamab deruxtecan-dlnk (Datroway®): Approved for the treatment of adult patients with locally advanced or metastatic EGFR-mutated NSCLC who have received prior EGFR-directed therapy and platinum-based chemotherapy.
What are some side effects of EGFR-positive NSCLC treatments?
Side effects of the EGFR drugs vary by drug and by patient. Some common side effects of EGFR drugs include:
- Rash
- Itching
- Diarrhea
- Mouth sores
- Loss of appetite
- Inflammation around nails
- Weakness
- Cough
- Alopecia
- Constipation
- Nausea
Among the more serious but less common side effects of EGFR drugs are:
- Interstitial lung disease (group of disorders that causes scarring of the lungs)
- Vision toxicities (decreased vision due to a given drug)
- Severe skin lesions
- Cardiomyopathy (heart muscle disease)
- Cough
Get more tips on managing treatment-related side effects.
Will targeted therapy cure my EGFR lung cancer?
While your cancer may be managed with a targeted therapy for years with limited or no progression (cancer spread), it will not cure your cancer.
Unfortunately, after an initial positive response, many people with EGFR+ NSCLC develop drug resistance to the therapy, meaning the drug stops working and their cancer begins to grow and spread again.
Luckily, EGFR-positive NSCLC has a number of FDA-approved drugs, and patients are often able to switch to a different drug once one stops working. Your doctor will likely recommend a rebiopsy (a second biopsy of your tumor tissue) to determine what is causing your resistance before recommending another drug
Many research efforts are underway to understand drug resistance and find ways to combat it. For example, researchers are looking at various drug combinations (using multiple drugs to treat your cancer) that may help delay or prevent resistance from happening.
Can people with EGFR-positive lung cancer receive immunotherapy?
EGFR-positive NSCLC does not seem to respond to immunotherapy, which is a type of treatment that helps your immune system find and attack lung cancer. Researchers are actively exploring ways to open up immunotherapy options to patients with biomarker-driven NSCLC.
What are other treatment options for EGFR-positive NSCLC?
Other treatment options include chemotherapy, radiation therapy, and clinical trials. Each time you have to make a new treatment decision, be sure to talk to your doctor about your options, including discussing your goals for the treatment and possible side effects of your options, to determine the best treatment option for you.
Talk with your doctor about your EGFR mutation and your treatment options
Talk with your doctor to understand your EGFR mutation and type of cancer, and to make a treatment plan together. Bring a pen and paper or use your cell phone to write down your questions and your doctor’s answers. You might start with these questions:
- Did I have comprehensive biomarker testing – which biomarkers did you check for? If not, can you order it?
- What are my treatment options?
- Do I qualify for a clinical trial testing a new treatment for EGFR mutation?
- Is immunotherapy an option for me?
- What are the possible side effects of these treatments?
- Do I have any other biomarkers that may change my treatment options?
- What will we do if my treatment stops working?
Find more questions to guide your discussions with your doctor.
How is EGFR treated?
The treatment options for non-small cell lung cancer vary based on where the lung cancer is located within the lungs and the stage (whether the cancer has spread or not) of your EGFR diagnosis. You will work with your doctor to decide on the treatment that is best for you, based on your type and stage of EGFR and your biomarkers.
Below are some common treatment options for people with EGFR lung cancer.
Chemotherapy
Chemotherapy is a treatment that uses drugs to stop the growth of cancer cells. Chemotherapy also attacks healthy cells.
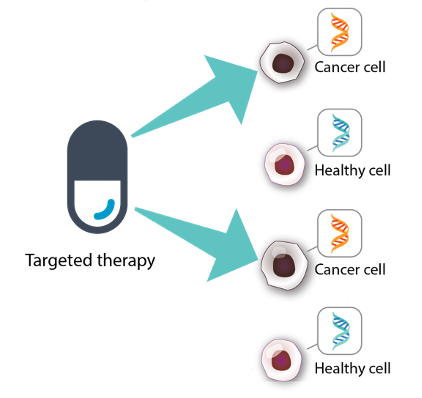
Targeted therapy
Targeted therapy is a type of treatment that finds and attacks certain parts of cancer cells and the signals sent to cancer cells that cause them to grow uncontrolled. They prevent the growth of cancer cells only, and do not harm healthy cells.
A targeted therapy works to control a specific biomarker, so it can only treat patients with that specific biomarker.
Targeted therapy attacks cancer cells only, and leaves healthy cells alone
Immunotherapy
Immunotherapy is a type of treatment that helps your immune system find and attack lung cancer. It is already a standard of care treatment for patients with EGFR.
Many of the FDA-approved immunotherapies target another type of biomarker called PD-L1. Because research has found PD-L1 biomarkers along with EGFR mutations in patient tumor samples, clinical trials are now learning if immunotherapy works to treat different types of EGFR mutations. Other clinical trials are underway to look at combinations of targeted therapy and immunotherapy for treatment of various NSCLC subtypes.
Clinical trials
Talk with your doctor about taking part in a clinical trial for a new, possible treatment. Clinical trials are an important option for people with EGFR because the newest treatment approaches, not available otherwise, are being tested in them.
For example, researchers are currently testing cancer vaccines and lab-made immune system cells (cells that find and attack cancer) that will target driver mutations in EGFR.
Palliative care
Palliative care does not treat lung cancer directly. The goal of palliative care is to improve your quality of life while receiving treatment. It prevents or treat the symptoms and side effects of the disease and its treatment. It also helps you manage emotional, social, practical, and spiritual problems you may face.
Talk to your doctor to learn more about your type of cancer and treatment options
Talk with your doctor to understand your type of lung cancer and to make a treatment plan together. Bring a pen and paper or use your cell phone to write down your questions and your doctor’s answers.
You might start with these questions:
- What type of lung cancer do I have?
- What is the stage of my lung cancer and what does this mean for my treatment?
- Did I have comprehensive biomarker testing – which biomarkers did you check for? If not, can you order it?
- What are my treatment options?
- What biomarkers do I have?
- Is immunotherapy an option for me?
- What is a clinical trial and should I enter one?
- What are the possible side effects of these treatments?
Find more questions to guide your discussions with your doctor.
Tip: Print these questions out and fill in the answers with your doctor. Having this sheet on hand can help you to remember the conversation and plan for any next steps.
Important Advice for Patients with Newly Diagnosed EGFR-Positive Lung Cancer
In this video, lung cancer expert Dr. Zosia Piotrowska speaks with Dr. Amy Moore, LUNGevity’s VP of Global Engagement and Patient Partnerships, to discuss the key information everyone with newly diagnosed EGFR-positive lung cancer needs to know. Dr. Piotrowska is a researcher and thoracic oncologist at Massachusetts General Hospital Cancer Center.

